
CHARACTERISTICS AND PROPERTIES OF GRAPHENE
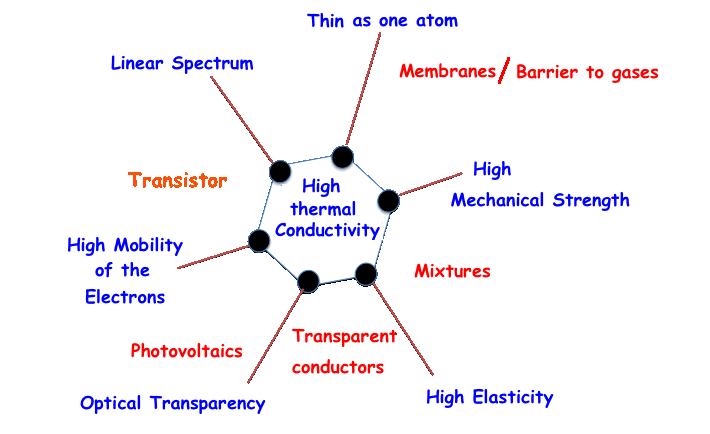
The electrical, optical and mechanical properties of graphene can be summarized as follows:
the electrical conductivity, at ambient temperature, is better than the electrical conductivity of the copper (nowadays all the electrical circuits have copper conductors);
the speed of the electrons is only 300 times lower than the speed of the light;
the mechanical strength is 42 N/m, practically this material is one hundred times stronger than the steel;
the thermal conductivity is the highest measured until today;
the elasticity is of 20%, it means that graphene can stretch up until 20% of its initial length;
the optical transparency is very good, graphene absorbs only 2,3% of the light;
A sheet of graphene has a thickness about 0,35 nm (1 nm = 10-9 m).
All the amazing properties of this extraordinary material are related with the perfect distribution that the nature has given to the carbon atoms of the graphene; these atoms are strongly bound together but the bonds, at the same time, are enough flexible to allow an elasticity of 20%.
The structure of graphene allows the electrons to move freely in the material, along considerable distances, so that they provide to the material an electrical conductivity higher than the conductivity of the standard conductors. In practice, the high electrical conductivity of graphene is due to the four valence electrons of the carbon atom; three of them are engaged in the planar bonds (sp2 hybridization) and they determine the two-dimensional structure of the graphene; instead the fourth electron, that is located in the “p type” atomic orbital, that is not involved in the hybridization process, is able to move almost freely in the structure of the graphene through long distances. The electrical conductivity of the graphene is higher (10 - 100 times) than that of the traditional conductors, where the electrons clash with the atoms and dissipate energy in heat.
The most promising applications of graphene are connected in particular to Electronics, not only due to the extraordinary electrical properties but also because, in the same material, the characteristics required to an ideal conductor are present, such as:
the low resistivity (~1,0·10-8 Ωm);
the high density of current that can flow in the material (>108 A/cm2);
the high thermal conductivity. The thermal conductivity of graphene (~5000 Wm-1K-1) has been recently measured at ambient temperature and it is much higher than all the values observed for other carbonaceous structures: nanotubes, graphite and diamond. On the contrary, the single-layer, deposited on a substrate of SiO2, shows a conductivity of 600 Wm-1K-1, which is approximately a factor 10 lower than the value of the conductivity in the free state; this value is, in any case, higher than the conductivity of the metals, such as copper (380 Wm-1K-1) and silver (430 Wm-1K-1).
Look at the video: Graphene on The One Show